Beneficence Belmont Report
The Belmont Report beneficence principle is the obligation of a researcher to do no harm to a participant. The Belmont Report attempts to summarize the basic ethical principles identified by the Commission in the course of its deliberations.

Comparison Of The Application Of The Beneficence Principle As A Download Scientific Diagram
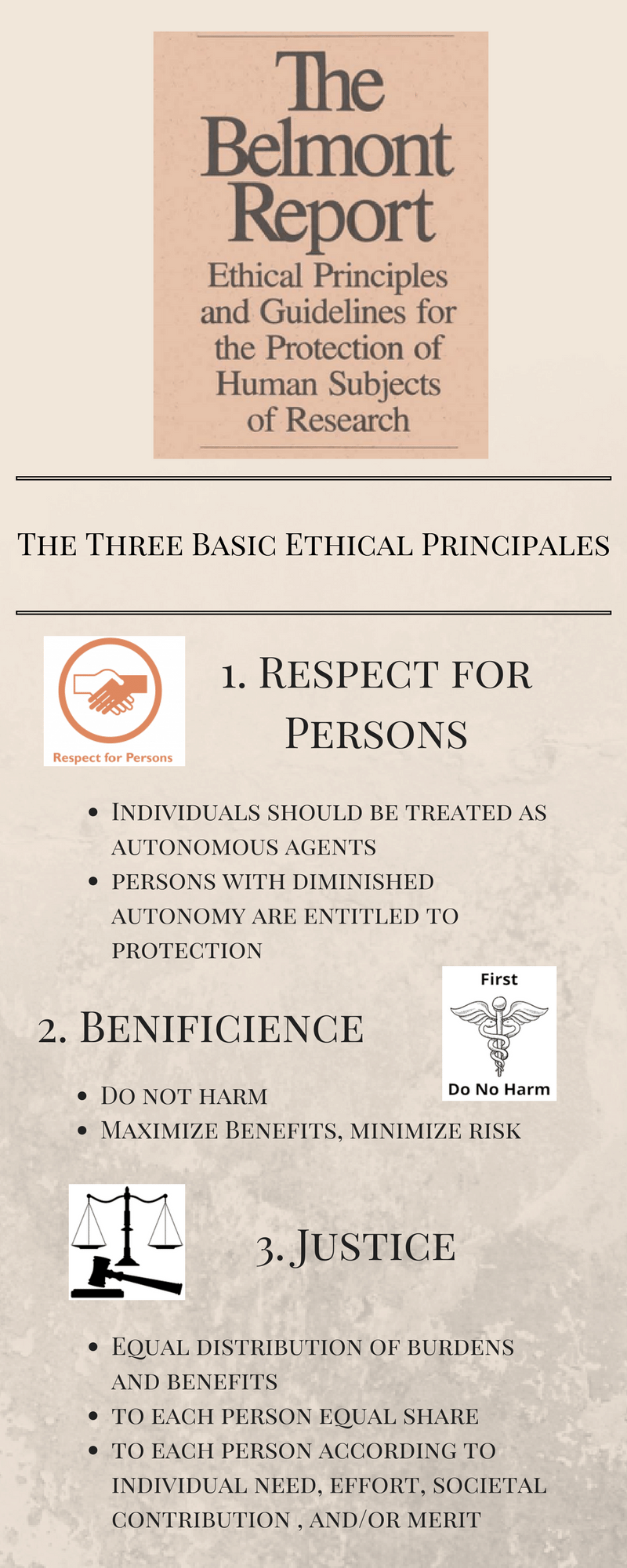
Similarly the three ethical principles laid forth in the Belmont Report ask providers to prioritize respect for persons beneficence and justice in their daily practice.

. Justice is the ideal distribution of risks and benefits when scientists conducting clinical research are recruiting volunteer research participants to participate in clinical trialsThe concept gives guidelines on how scientific objectives and not membership in either a privileged or vulnerable population should. In 1981 Beauchamp and Childress built on this work and applied it to health care in the first edition of their book. Subsequent regulations through the Department Health and Human Services require all institutions that.
Respect for persons includes the recognition of personal dignity and autonomy and provides the need to obtain voluntary. 1 Brutal or inhumane treatment of participants is never morally justified 2 Should determine whether use of human subjects is necessary at all - reduce risk 3 Significant risk of serious risk of impairment - review. Following a series of highly-publicized medical and behavioral research scandals the 1974 National Research Act and 1979 Belmont Report set a new agenda for research ethics in the US that rested on principles of beneficence respect for persons and justice.
Researchers must minimize harm to research participants and maximize benefits of research. To interpret these ethical principles IRBs would ultimately look to an unlikely amalgam of concerned healthcare professionals scientists theologians and philosophers. In research ethics justice is the fair selection of research participants.
Read about the four principles of biomedical ethics. Such tenets may allow doctors care providers and. The Belmont Report attempts to summarize the basic ethical principles identified by the Commission in the course of its deliberations.
The basic ethical principles. The Belmont Report is one of the leading works concerning ethics and health care research. While is it critical for providers of medical care to uphold these tenets frequently situations arise where it is.
Today these guidelines provide a platform for the protection of human research subjects including the. These values include the respect for autonomy non-maleficence beneficence and justice. Many of these guidelines are based on the Belmont Report pdf.
It is the outgrowth of an intensive four-day period of discussions that were held in February 1976 at the Smithsonian Institutions Belmont Conference Center supplemented by the monthly deliberations of the Commission that were held over a. Ethical Principles and Guidelines for the Protection of Human Subjects of Research The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research April 18 1979. First that individuals should be treated as autonomous agents and second.
Belmont Report National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research 1979 which identified the principles of respect for persons beneficence and justice in human subjects research. Beneficence -- requires that experiments do not harm research subjects and that researchers minimize the risks for subjects while maximizing the benefits for them. In its 1978 Belmont Report the Commission stipulated that in reviewing research proposals IRBs should be guided by three basic ethical principles.
The Belmont Report has provided the basic moral framework for research ethics in the United States. Its primary purpose is to protect subjects and participants in clinical trials or research studies. Medical ethics is based on a set of values that professionals can refer to in the case of any confusion or conflict.
The guiding ethical principles of the IRB - respect for persons beneficence and justiceare embodied in the Belmont Report. What 5 assessments of justifiability of conducting human research should be conducted according the the Belmont Reports Principles of Beneficence. Autonomy beneficence non-maleficence and.
Congress with a charge to discover and publish the basic principles of human research ethics and also to consider the boundaries between biomedical research and accepted medical practice. Medical ethics is an applied branch of ethics which analyzes the practice of clinical medicine and related scientific research. See examples of bioethical principles in action.
It is the outgrowth of an intensivefour-day period of discussions that were held in February 1976 at the Smithsonian Institutions Belmont Conference Center supplemented by the monthly deliberations of the Commission that were held over a. This included the principle of respect for all people which demands that all individuals should be provided with an opportunity to make up their minds about participating in the research after they are privy to all information regarding. These principles are respect for persons beneficence and justice.
These include guidance documents and frequently asked questions FAQs addressing various topics findings in the form of OHRP letters addressing regulatory issues and other media including. The Hippocratic Oath instructs physicians and other medical providers to first do no harm. Study with Quizlet and memorize flashcards containing terms like Which of the following are the three principles discussed in the Belmont Report an example of how the Principle of Beneficence can be applied to a study employing human subjects incorporates at least two ethical convictions.
This report consists of 3 principles. This commission was established in 1974 by the US. Justice -- requires that all forms of differential treatment among research subjects be justified.
These abuses served as the impetus for the establishment of the Nuremberg Code Declaration of Helsinki and the National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research 1974 and the resulting Belmont Report. Today we are highly influenced by the Belmont Report that outlines the ethical principles that all researchers must be sure to follow. One of these the Belmont Report published in 1978 described the three ethical principles on which the procedural requirements of the Common Rule are based.
OHRP has published a variety of policy and regulatory guidance materials to assist the research community in conducting ethical research that is in compliance with the HHS regulations. Respect for persons beneficence and justice. National Center for Biotechnology Information.

From The Belmont Report To The Code Of Federal Regulations
Historical Context And Economic Issues Ethical Legal And Societal Implications Of Biotechnology

Extensions Of The Belmont Report S Principles Based On The Guidelines Download Scientific Diagram

Comparison Of The Application Of The Respect For Persons Principle As A Download Scientific Diagram
0 Response to "Beneficence Belmont Report"
Post a Comment